Research
Translational Neuroscience and Neurology
Gliovascular dysfunction in aging and neurodegeneration
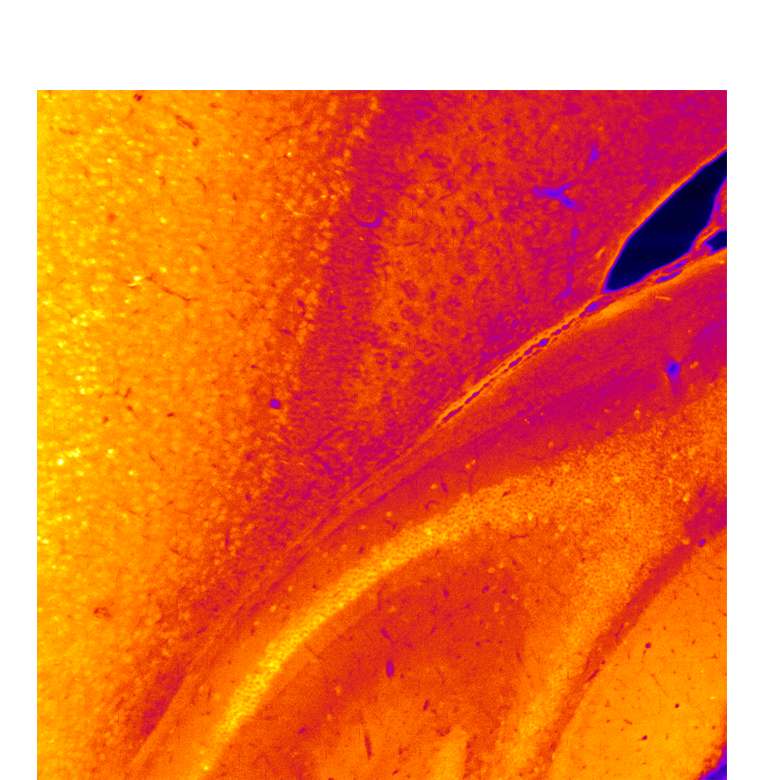
Brain health critically depends on gliovascular cells, each serving crucial homeostatic functions to support the operation of neural circuits. Over the past decades, accumulating evidence point to causal roles of gliovascular dysfunction in various neurodegenerative diseases. Our team adopts a multimodal approach combining genetic manipulation, molecular assays, in vivo functional and structural imaging to uncover how aging and neurodegenerative disease-related genetic mutations cause gliovascular dysfunction.
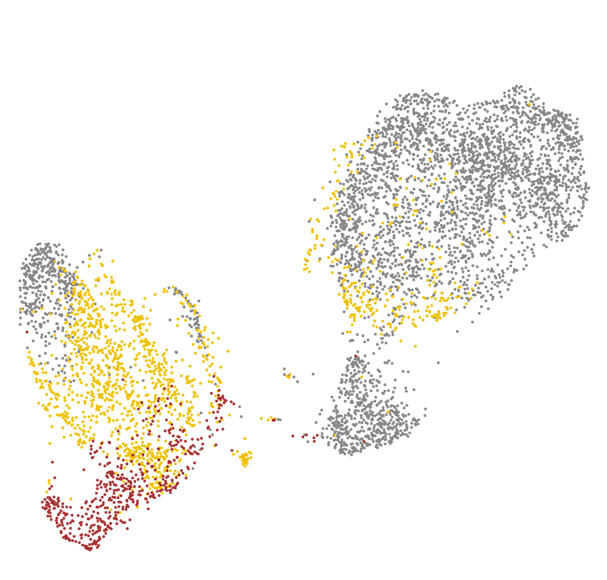
We are also interested in the development of disease-modifying therapeutics, as exemplified by our recent work identifying glucagon-like peptide-1 receptor agonist (GLP-1RA) as a promising potential treatment for age-related vasculopathy (Zhao et al., Nature Communications, 2020 Sep 4;11(1):4413), which can also reverse transcriptomic aging signature in many brain cell types in a genome-wide manner (Li et al., Communications Biology, 2021 Jun 2;4(1)656; US/PCT patent application filed), implicating broad potential applicability of GLP-1RAs for the primary prevention and secondary treatment of age-related neurodegeneration. Currently, we are also working closely with clinical teams to conduct pilot clinical trials inspired by our pre-clinical discoveries.
Systems Neuroscience
Neural circuits for sensory processing and behavior
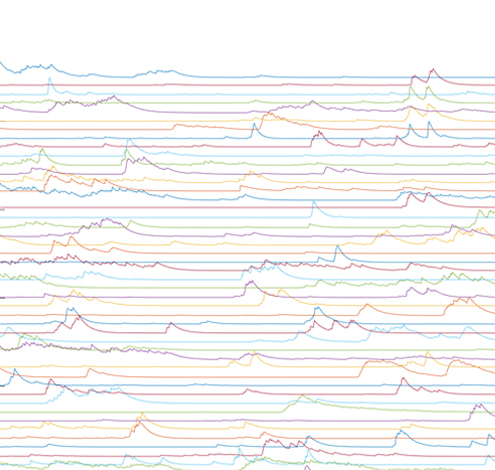
We aim to attain a better understanding of the neural circuit basis of sensorimotor behavior and learning. To achieve this goal, we seek to uncover how neurons across different brain regions encode behavioral task-relevant information, such as sensory features and predicted outcomes, to instruct decision making and the generation of motor commands.
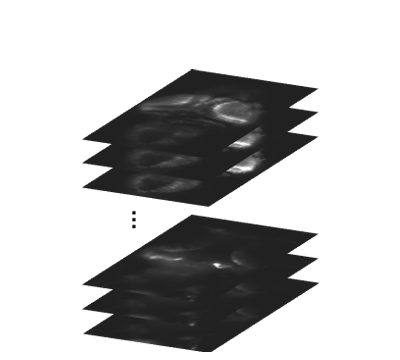
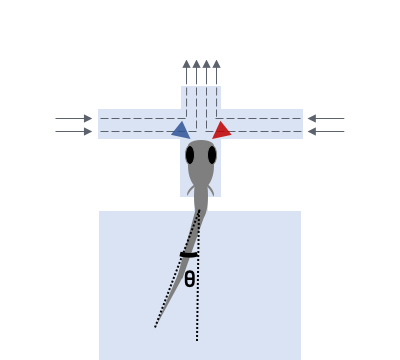
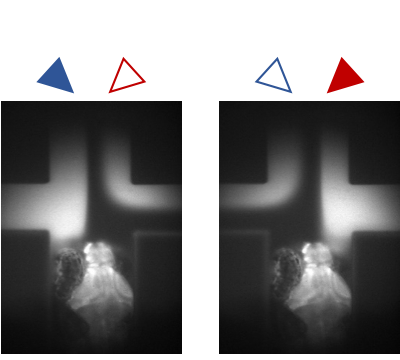
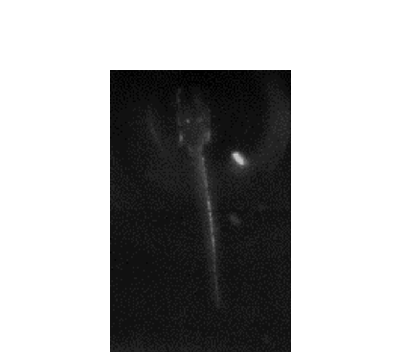
In our lab, we develop platforms on which (i) larval zebrafish are presented with spatiotemporally precise chemical stimulation (Sy et al., Nature Communications, 2023), or (ii) mice learn to execute complex forelimb motor and general locomotor tasks in response to visual cues, while neuronal activities are recorded from different brain areas by light sheet or multiphoton microscopy. Combined with circuit tracing techniques, we are studying the neural coding of (i) ecologically significant odors and odor mixtures in larval zebrafish, and (ii) task-related information along pathways connecting visual areas to association and motor areas in mouse. In the future, we plan to extend our investigations to animal models of neuropsychiatric diseases.
Methods and Tools
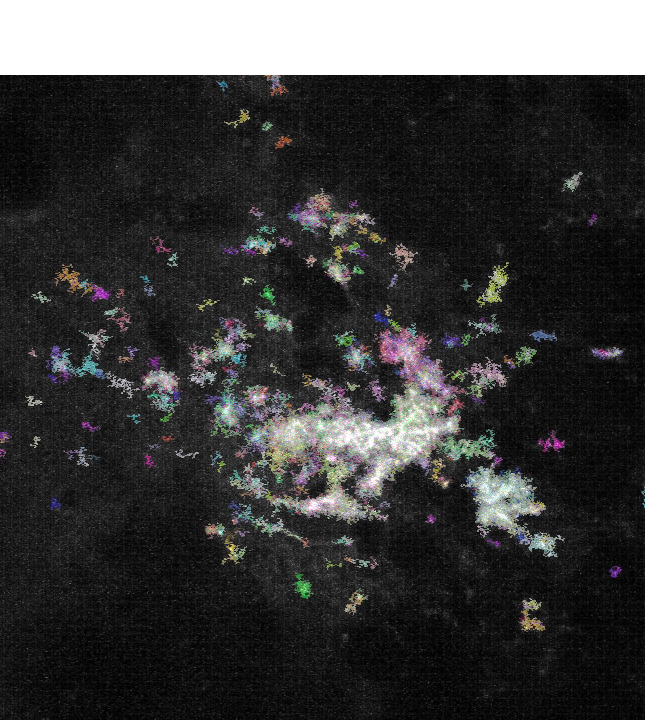
Bottlenecks in neural circuit and brain disorder investigations are often due to methodological limitations. We are actively developing imaging tools for pre-clinical investigations and potential clinical applications, emphasizing new methods for high-throughput three-dimensional molecular profiling in intact tissues. These techniques will not only empower our investigations of neural circuit functions in health and disease, but also benefit other fields of biomedical sciences.
Our recent works include the development of a new hydrophilic tissue-clearing chemical cocktail solution, and a method for the thermostabilization of antibodies that permit heat-accelerated deep immunostaining of intact organ tissues (Lai et al., Nature Methods, 2022; Yau et al., Cell Reports Methods, 2023; three US/PCT patent applications filed). Currently, we are attempting to further advance multiplexed deep immunostaining and in situ RNA imaging methods, which we plan to combine with other techniques to tackle systems and translational neuroscience problems.
Bottlenecks in neural circuit and brain disorder investigations are often due to methodological limitations. We are actively developing imaging tools for pre-clinical investigations and potential clinical applications, emphasizing new methods for high-throughput three-dimensional molecular profiling in intact tissues. These techniques will not only empower our investigations of neural circuit functions in health and disease, but also benefit other fields of biomedical sciences.
Our recent works include (i) the development of a new hydrophilic tissue-clearing chemical cocktail solution, and a method for the thermostabilization of antibodies that permit heat-accelerated deep immunostaining of intact organ tissues (Lai et al., Nature Methods, 2022 (accepted); three US/PCT patent applications filed), and (ii) a new method for the room-temperature synthesis of metal halide quantum dots – an attractive material as potential fluorophores (Lai et al., ACS Applied Nano Materials, 2022). Currently, we are attempting to further advance multiplexed deep immunostaining and in situ RNA imaging methods, which we plan to combine with other techniques to tackle systems and translational neuroscience problems.
Neural circuits underlying sensorimotor behaviour
We are currently investigating neural circuits underlying sensorimotor behaviour in two model systems:
i. Mus musculus (Mouse)
To survive in the wild, animals must be able to associate sensory cues with the need to perform corresponding motor responses. These processes involve coordinated neural activity and signal flow across primary sensory, association and motor areas. Often, sensorimotor tasks also necessitate the acquisition of novel motor sequences from an existing motor repertoire. To understand sensorimotor learning, we must uncover how neuronal populations across different brain regions encode various aspects of task-relevant information, such as sensory features and predicted outcomes, and instruct the generation of complex sequences of motor commands.
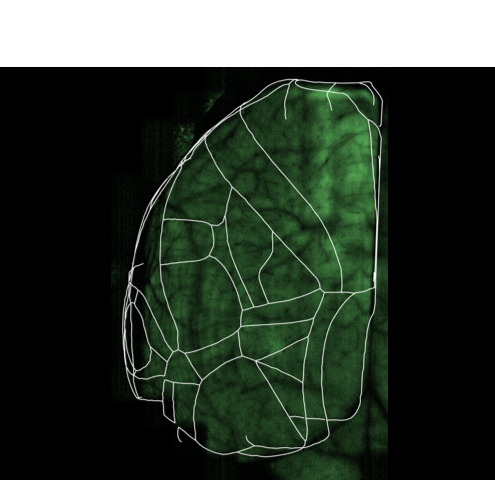
In our lab, we develop fully automated behavioural and stimulation platforms on which mice learn to execute complex forelimb motor and general locomotor tasks in response to visual cues, while neuronal activities are recorded from different cortical areas by multiphoton microscopy. Combined with circuit tracing techniques, we study the rules governing the encoding of distinct task-related information along pathways connecting higher visual areas to association and motor areas.
ii. Danio rerio (Zebrafish) larvae
Several days post-fertilization, zebrafish larvae already exhibit an incredible repertoire of innate behaviors including chemosensory behaviour, alongside phototaxis, oculomotor and optokinetic responses, prey capture and various escape responses. As a model organism for neuroscience, larval zebrafish offers the distinct advantages of relatively high optical transparency and more tractable total number of neurons in the brain (in the order of 105), permitting the interrogation of brainwide activities mediating sensorimotor transformation with cellular resolution. We are interested in fundamental questions of how neural circuits in the brain process survival-relevant sensory cues to drive motor response.
To this end, we have constructed an integrated microfluidics-behavioural and brainwide imaging platform, to precisely manipulate the sensory environment of zebrafish larvae while performing volumetric whole-brain imaging of neuronal activities. Currently, we are characterizing circuit principles governing the integration of chemical signals for guiding chemosensory navigation.
Gliovascular dysfunction in aging and neurodegeneration
Brain health critically depends on diverse gliovascular cells, each serving crucial homeostatic functions in the maintenance of neural circuit functionality. Over the past decades, accumulating evidence point to causal roles of gliovascular dysfunction in various neurodegenerative diseases. We adopt a multimodal approach combining genetic manipulation, molecular assays, in vivo functional and structural imaging to uncover how aging and neurodegenerative disease-related genetic mutations cause gliovascular dysfunction. We are also interested in the development of disease-modifying therapeutics, as exemplified by our recent work identifying glucagon-like peptide-1 receptor agonist (GLP-1RA) as a promising potential treatment for age-related vasculopathy (Zhao, Li and Vong et al., Nature Communications, 2020 Sep 4;11(1):4413), which can also reverse transcriptomic aging signature in many brain cell types in a genome-wide manner (Li, Chen, Vong and Zhao et al., Communications Biology, 2021 Jun 2;4(1)656), implicating broad applicability of GLP-1RAs to the primary prevention and secondary treatment of age-related neurodegeneration.
Contact Us
Li Ka Shing Institute of Health Sciences
Rm 502, Li Ka Shing Medical Sciences Building
Prince of Wales Hospital, Shatin, Hong Kong
Email: ho.ko@cuhk.edu.hk